HYDROGEN FUEL CELL MATERIALS
TFP has been developing and optimising material for fuel cell GDLs since 1988, working alongside some of the leading companies in the industry. This long term commitment to fuel cell development and growth has facilitated a comprehensive understanding of the necessary material characteristics to achieve a high performance GDL substrate.
The result is a range of carbon veils and mats which can be tailored to suit the requirements of both stationary and portable fuel cell systems. To date our nonwovens have been used successfully as GDLs in proton electrolyte membrane (PEMFC), phosphoric acid (PAFC) & direct methanol (DMFC) fuel cells.
In addition our subsidiary TFP Hydrogen Products also offer solutions for fuel cell technologies, ranging from catalyst powders to components for electrolysers which enable the generation of green hydrogen fuel from water electrolysis.
TFP Hydrogen Products' high-performing range of catalyst powders are used in the production of membrane electrode assemblies (MEAs), gas diffusion electrodes (GDEs) and catalyst coated membranes (CCMs), they have been specially developed for fuel cell applications, meeting market requirements and demand. These materials include Pt/C catalysts and a new transient-tolerant anode catalyst.
WHAT IS A FUEL CELL?
A fuel cell is an electrochemical device that combines hydrogen (or hydrogen-rich) fuel and oxygen to produce electricity, heat and water. It is much cleaner and more efficient than an internal combustion engine (ICE) as it does so without burning the fuel. Essentially more of the fuel is converted into electricity and less into heat.
The active components of a fuel cell are combined in the membrane electrode assembly (MEA), which is composed of an anode, a cathode and an electrolyte membrane. Hydrogen fuel is supplied to the anode, and oxygen to the cathode, via the bipolar plates. The anode is coated with a catalyst which accelerates the conversion of hydrogen to protons and electrons. The electrolyte membrane is selective to protons only, allowing them to pass through to the cathode. The electrons must move via an external circuit generating an electrical current and excess heat. At the cathode the protons, electrons and oxygen combine to generate a water molecule, the only by-product of the process.
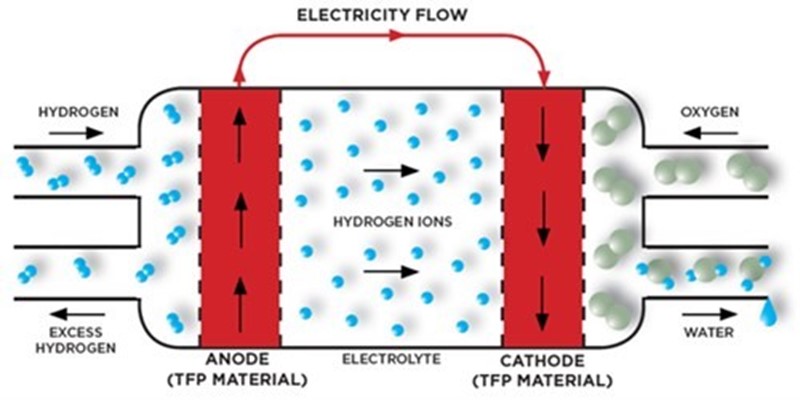
Individual fuel cells can be compiled to form stacks, which in turn can be combined into larger systems. This means that fuel cell systems vary significantly in both size and power, ranging from portable systems for transportation to large scale installations that provide electricity for high energy demand applications such as schools and hospitals.
THE BENEFITS OF FUEL CELLS
Fuel cells offer a number of advantages compared to batteries or internal combustion engines, these include:
- Improved Efficiency - Higher levels of efficiency. If both the electricity and heat generated can be utilised, a fuel cell can be considered up to 85% efficient in converting hydrogen to energy. By comparison, the best ICEs are less than 40% efficient.
- Reduced Noise & Maintenance - Fuel cells are very quiet in operation and have fewer moving parts than traditional generators. This makes them both more reliable and simpler to maintain.
- Continuous Power - Unlike batteries, fuel cells do not need to be periodically recharged. They continue to produce electricity for as long as fuel is provided. This gives fuel cell electric vehicles (FCEVs) 2 key benefits over battery electric vehicles (BEVs). Firstly, the filling time is short - as little as 5 minutes, significantly less than a BEV charge time of between 30 minutes and 12 hours. Secondly, once filled a FCEV can travel on average 250 miles - a lot further than the 114 mile per charge average for a BEV. A further advantage in larger vehicles particularly is the weight saving of the power source; larger batteries can be very heavy in comparison with a fuel cell equivalent.
- Clean & Renewable - The only by-product of a hydrogen fuel cell is water, eliminating the pollutants associated with burning fossil fuels. Their fuel source, hydrogen, can also be generated from renewable energy, further increasing green credentials. In addition, the fact that they do not need to use oil or gas as fuel also reduces economic dependence on these resources and creates greater energy security.
- Flexibility in Function - Fuel cell systems vary in size, ranging from large scale installations providing electricity for buildings, to portable fuel cells for applications including transport. Some fuel cells can also operate at lower temperatures, making them ideal for military applications as they have low heat transmission.
SOLUTIONS FOR HYDROGEN FUEL CELLS

Electrical Conductivity
Nonwovens which offer tuneable electrical conductivity for a wide range of applications.
Learn More 